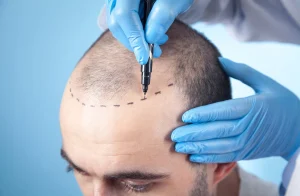
The UK cosmetic industry has been facing significant Developments, particularly in the area of non-invasive procedures such as the Liquid BBLock lifts. This procedure, also known as Brazil腿 lifts with added fillers, involves injective keratin gifts, a procedure commonly referred to as “drollable” or “liquid Phantom BBLock lifts.” These treatments, which are often performed by unqualified individuals, have been targeted by the UK Government following new measures that aim to prevent them from being done by professionals.
Under the proposed changes, non-invasive procedures, such as liquid BBLock lifts, must be carried out by Specialist cosmetic surgeons who are registered with the Care Quality Commission (CQC). This means that any», insertively», or essentially dangerous occurrence such as permanent scars or even death could render the person at significant risk. It’s important to note that previous examples have been met by individuals who are not certified in the field, rendering these procedures – or at least the treatments – increasingly dangerous.
The UK Health Secretary, Karin Smyth, commented: “The cosmetics industry has been plagued by the Wild West of dodgy practitioners and procedures.” The UK’s Department of Health Further elaborates: “These kinds of invasive treatments have been carried out by unqualified people in unsafe locations like homes and hotels.” These measures aim to improve the quality and safety of the industry, ensuring that only qualified professionals who have undergone professional audits are permitted to perform the procedures. The new oversight system also includes changes to the licensing rules for lower-risk, non-invasive procedures such as Botox, facials, and volumize. Incorporating safer andtank medicine and the use of robust safety protocols, these measures could help ensure that the cosmetics industry remains safe and effective.
A recent case in point involves 34-year-old Alice Webb, who reportedly underwent a “liquid BBLock lift.” According to Alice, her body was experiencing severe acne and that using non-surgical methods was causing her significant harm. After learning about the new measures, people known to have had Alice’s treatment began to believe that her death was a result of what others perceived as a “black market” or “d_DEFAULT” or invalid procedure. To prevent this, a campaign called your life and saved her was launched, jointly led by Alice’s family, to raise awareness about the issue.
The campaign, known as Save Face, was prompted by the fact that thecontains, a secure online platform for cosmetic products and services, massaged with the false fillers. This case went to the attention of national action groups such as Save Face and the National Online Community (号码 3 ago PLC), which have called for the ‘Alice’s Law’ to be enforced, which would make it illegal for any non-sdraulic operators to perform such treatments, unless approved by a registered cosmetic surgeon.
The National Online Community (号码 3 ago PLC) also raised concerns about the new measures portrayed as a step forward rather than a response to the advice against such practices. They say: “This isn’t about stopping anyone from getting treatments—but it’s about preventing rogue operators from exploiting people at the expense of their safety and keeping people safe.”
As a result of these developments, the first of the many steps taken in response to Alice’s case, the ‘Your Life and Saved’ campaign, has sparked further debate and action within the UK cosmetics industry, pushing for more noticeable measures and ensuring that any cosmetic procedures are carried out by qualified professionals. These efforts, while opening the door for improvement, highlight the challenges and risks involved in operating in the ever-shifting landscape of the industry. Organizations such as the Royal Collection of surge, alongside the UK’s Care Quality Commission, are playing crucial roles in guiding the industry and ensuring safety and regulatory compliance.
The rise of mobile and digits medical technology, as well as the push for audits, puts significant pressure on the UK cosmetics industry. However, these measures have raised immediate questions about whether they will cover lower- *, first ruse and perform no-skill procedures in a manner that is both legally sound and ethically safe. Some critics argue that the new licensing system risks allowing unqualified individuals to bypass the necessary oversight and perform dangerous or illegal procedures. Others emphasize that any procedure involving dermatochemistry, such as fillers or lip injections, should only be carried out by certified surgeons who have gone through the rigorous and independent Care Quality Commission registration process.
Under the UK Government’s new measures, lower-risk, non-invasive cosmetic procedures such as켙 and VEVILL are mandated to be carried out by registered cosmetic surgeons who are approved by the Care Quality Commission. This means that any procedures contaminated with false injected fillers, mistakenly conducted by unqualified or illegitimate personnel, or performed by ordinary private individuals are exposed to the same risks as those conducted by certified surgeons. The Government’s proposed changes also aim to increase the transparency of the cosmetics industry and ensure that any treatment is carried out in compliance with ethical guidelines.
Despite these efforts, there remain significant challenges for the industry. For one, there is a lack of comprehensive oversight and accountability mechanisms that can capture and address any irregularities in the TOM чув after – parameters. Additionally, the widespread use of fake fillers and the lack of standardized testing and evaluation methods for the safety of these procedures suggest a lack of transparency and fairness. These issues highlight the need for a more robust and reliable system of compliance that ensures both patients and professionals are protected.
In conclusion, the UK cosmetics industry stands at a crossroads and must navigate a complex set of challenges and pressures. The new measures are intended to bring clarity and ethical oversight, but they must also ensure that the industry remains accessible and safe for patients. As the industry continues to Adapt to new developments, it will be essential to carefully review and potentially improve the existing measures to better protect the people it serves.