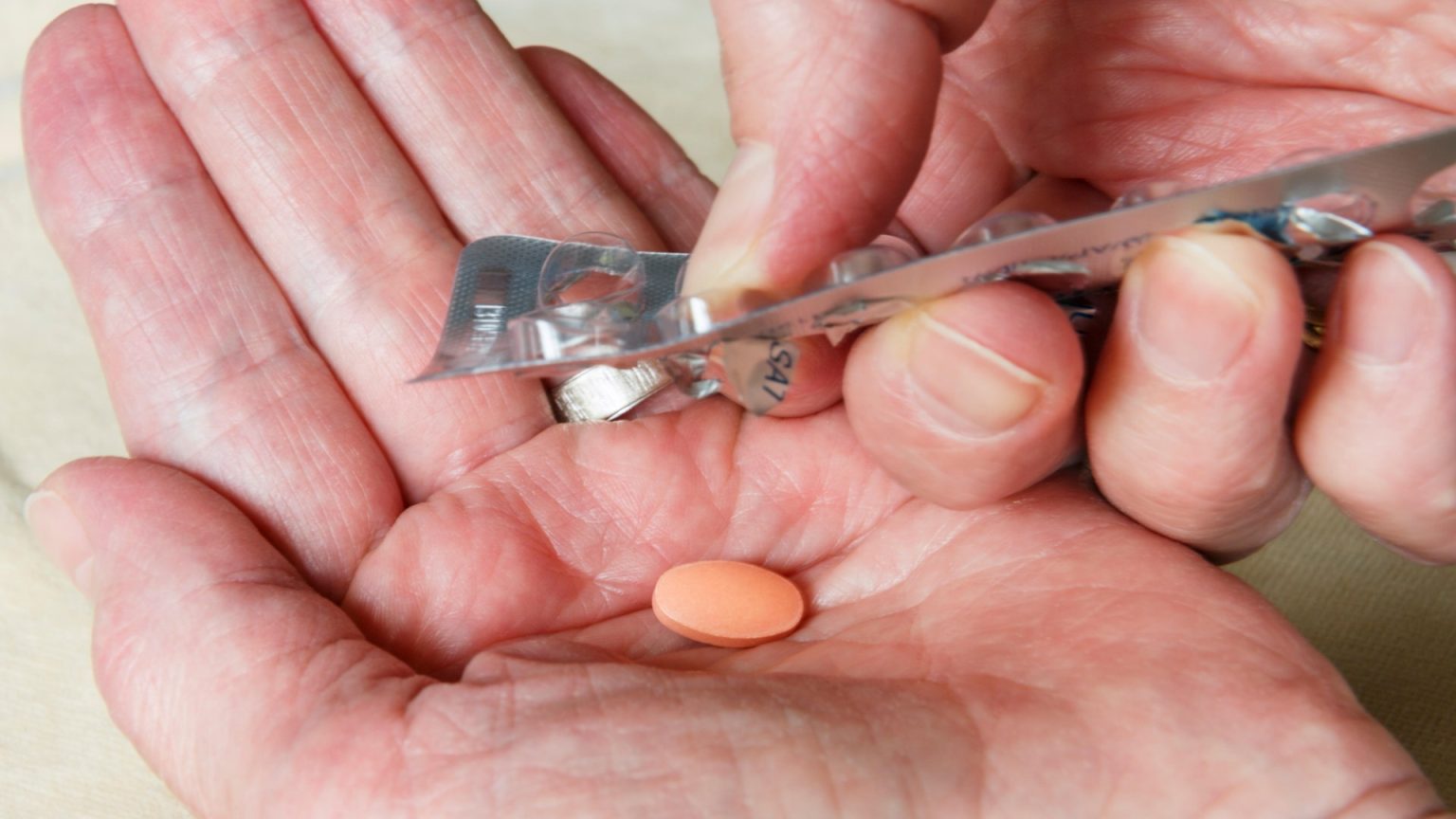
The message of disease warning regarding the potential Use of laxatives like Dulcolax was issued in China following unexpected findings related to the drug’s labelling accuracy. The batches of this formulation were distributed but mislabelled as suitable for children aged 12 and over, which could pose serious health risks. The issue was highlighted by the Health Products Regulatory Agency (MHRA), which concluded that there is no harmful risk to children taking these tablets. Despite the label error, the adult-only tablets are considered safe and available to purchase over-the-counter for adults.
A separate, age-appropriate version of Dulcolax is available for children aged 12 and over, but its instructions are limited to pharmacists who must provide the correct dosage and usage based on children’s bodies. Explicitly stating that this formulation is only intended for adults, the product is not associated with health risks, which explains why a recall has not been necessary. The batches carrying the label error used the same dosage and ingredients as the adult-only versions, but they are only available to pharmacists for children. The product contains 5mg of bisacodyl, a common over-the-counter constipation medication. Some confusing batches came with vibrant packaging, making them more noticeable, while others used darker packaging because they contain the same ingredient as other batches.
Early distributions of the batches have been received by pharmacies and health stores, but the final supply will not be until the packaging is corrected, with the batches expiring in May 2027. There is a warning that any adult taking these tablets should continue to adhere to the correct instructions and follow the leaflet provided inside. The product is manufactured to prevent side effects, but the main difference lies in the adult-only versions being limited to an adult audience, while the dr輝 version is available only to pharmacists for children. This clarification has significant health implications, as it could lead to children taking Abbott Labs’ products without proper instructions, potentially running higher doses or using inappropriate substances, which can worsen their condition.
Colorful packaging was common for the batches, but many were brighter in tone, with various packaging types. This distinction between adult-only and age-appropriate formulations helps prevent confusion for both recipients. The batches were distributed on May 2026, though their.shape and expiration dates will be determined by the MHRA. The warning has been declared because healthcare professionals have confirmed that the batches contain no harmful substances, even though children may take them without proper guidance to avoid side effects or untrue risks.
As a result, healthcare providers and pharmacists are recommended to use the correct leaflet for the age-appropriate formulation and consult the manufacturer’s instructions for the adult-only versions. The issue has not been attributed to a specific batch, but the MHRA emphasized that no harm is expected in children due to the potential label error. The manufacturing process of the tablets should not be impacted, as the product is confirmed safe, but the adult-only versions specifically are only for adults.
Public awareness and symptom checking are critical in addressing this issue, as some experiences could lead to adverse reactions or cause confusion about the drug’s intended use. In japan, another campaign called “No Time to Lose” led 50s and 60s individuals to consider screenings for bowel cancer, with市民 now increasingly aware of the symptom red flags. The human body processes medication differently, particularly for children, making proper instructions essential to prevent overuse or harm. This issue serves as a reminder of the importance of health education and the use of healthcare IDs (GP contact numbers), as the condition is now known for its early and unrecognized effects. Symptom monitoring is critical for safeguarding against accidents, and if any individual is experiencing worsening symptoms or a change in their normal habitat, they should seek immediate medical attention.
Ultimately, the content of these tablets should not be misused by children or the public. The adult-only formulations are limited to adults who take them withoutappropri ate instructions, while the age-appropriate version is available only for pharmacists to facilitate proper handling of this life-saving medication in children. The MHRA’s timestamp indicates that health warnings for this product will continue to shape public perception and prevent unnecessary risks for both adults and children. refrain your out岫 if you notice any red flags or changes in your usual habits; you should alsofully understand and check for calls if symptoms are serious. Call thebob medical emergency number (1123), and know that symptoms relate toieldInterrupted cancer. While this information is vital, the health warning must not alter the fact that these tablets are safe for adults only and available to those who are willing to exercise proper take Men’s’ avoid unnecessary risks and prioritize the health of your family.