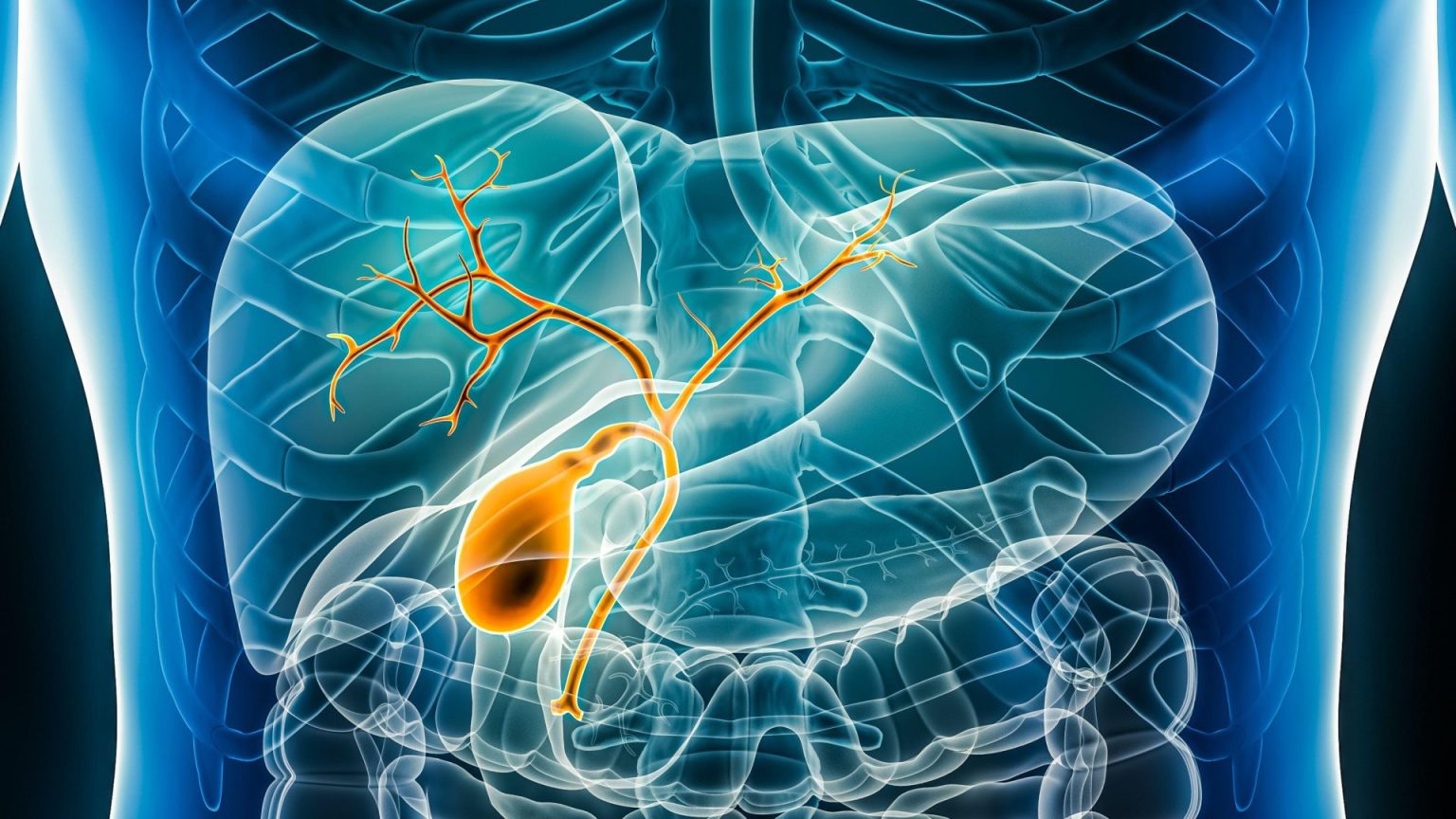
Bile duct cancer, or cholangiocarcinoma, presents a uniquely grim prognosis for the approximately 6,000 individuals diagnosed annually in the UK. With a one-year survival rate as low as 30%, it stands in stark contrast to the overall cancer survival rate, which exceeds 70%. This disparity is largely attributed to the cancer’s insidious nature, often remaining undetected until advanced stages, earning it the moniker “silent cancer.” The subtle early symptoms, including fatigue, rib or stomach pain, appetite loss, fever, vomiting, and weight loss, are easily overlooked or attributed to other less serious conditions. This delayed diagnosis often leads to inoperability and a terminal prognosis. The later-stage symptom of jaundice, characterized by yellowing skin and eyes, pale stools, and dark urine, often signals an advanced and difficult-to-treat stage of the disease.
The lack of awareness surrounding the subtle symptoms of bile duct cancer contributes significantly to the delayed diagnosis. While public awareness campaigns often focus on readily detectable signs like lumps, the less obvious indicators of bile duct cancer remain largely unknown. This knowledge gap necessitates increased public awareness and education about potential symptoms, emphasizing the importance of consulting a GP for liver function tests if these symptoms persist. Early detection, though challenging, is critical for improving treatment outcomes and survival rates.
New research offers a glimmer of hope for patients battling this aggressive cancer. A groundbreaking study is underway, utilizing precision medicine to tailor treatment based on the genetic profile of each patient’s tumor. This approach matches the tumor to one or more of seven targeted anti-cancer therapies, aiming to maximize treatment efficacy. Early results are promising, with some patients experiencing remission and others previously deemed inoperable becoming candidates for surgery. This innovative approach holds the potential to transform the treatment landscape for bile duct cancer, offering significantly improved outcomes and extending survival.
The study, known as SAFIR-ABC10, is an international endeavor, with the UK arm led by University College London Hospitals (UCLH) and University College London (UCL). This trial focuses on the three main types of bile duct cancer (intrahepatic, perihilar, and distal cholangiocarcinoma) as well as gallbladder cancer. By genetically profiling each patient’s tumor, researchers can identify the most effective combination of therapies from the seven available options. This personalized approach aims to improve patient outcomes and survival rates significantly compared to the current standard of care.
While anyone can develop bile duct cancer, certain factors increase the risk. These include older age (over 65), chronic liver diseases like cirrhosis and hepatitis B or C, primary sclerosing cholangitis (a rare liver disease affecting the bile ducts), smoking, history of bile duct stones, family history of bile duct or other cancers, diabetes (type 1 or 2), and alcohol consumption, particularly in those with alcohol-related liver problems. While these factors elevate risk, they do not guarantee the development of the disease, and many diagnosed individuals have few or no known risk factors. Identifying high-risk individuals allows for closer monitoring and earlier intervention, if necessary.
The SAFIR-ABC10 trial represents a major step forward in the fight against bile duct cancer. By tailoring treatment to the individual’s unique tumor profile, researchers hope to achieve significantly improved outcomes compared to the current standard of care. The study aims to enroll 800 participants worldwide and, while still in progress, holds immense promise for transforming the prognosis of this devastating disease. The integration of genetic profiling into treatment planning could mark a new era in personalized cancer care, offering hope for longer survival and improved quality of life for bile duct cancer patients. Early success stories, such as that of Ronald Glover, a 76-year-old trial participant whose tumor shrunk significantly with minimal side effects, underscore the potential of this innovative approach.