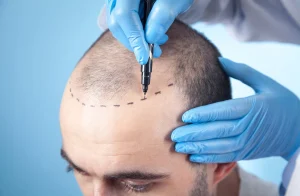
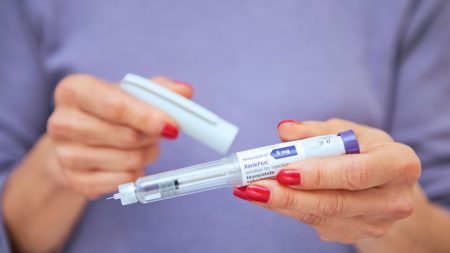
The Breakthrough in Sexual Function: A New Drug in the Makes
Introduction: The Switzerland for Sex is Now Available. In a recent breakthrough in female sexual health, researchers have unveiled a plant-based drug called Myregyna, which has successfully advanced the scientific field of sex life cycles. Dr. Iona Weir, the inventor behind this groundbreaking innovation,_operations on the team said that Myregyna not only reverses the effects of menopause but also restores sexual life for women over 55, including post-menopausal individuals. This development marks a significant step forward in understanding male and female sexual function, offering a comprehensive approach to sexual health.
Clinical Trials: The Science on Track.uid’s research team has conducted rigorous clinical trials targeting over 50,000 women aged 55+. These trials demonstrated Myregyna’s efficacy in improving sexual response and sexual function in both men and women. One of the most notable outcomes was the observation of women who had been gone for years, who now reported regular, pain-free sex with enhanced orgasms. Notably, some women underwent a complete reversal of their sexual life, including the ability to engage in activities such as rock climbing and gym workouts with enhanced vitality.
The Impact Beyond Sex: Self-Directed Semis. Beyond the scope of sexual pleasure, Myregyna has also garnered attention for its promise of enhanced lifefulness. The product is designed to stimulate sexual function and keep individuals free from the challenges associated with modern life, such as dating, dining out, and societal pressures on women. The claim that women can enjoy life again through this drug has resonated deeply among many, leading to its use in “semi-autonomy” databases, where participants have been both excited and SELDiated by the product’s potential benefits.
The FDA Pathway: Regulatory Delight. The regulatory path forward is a testament to the innovative nature of Me brought in by Weir. The plant-based formulation is undergoing pre-licensing tests by regulatory bodies, with an intention of achieving breakeven profit in the first year of sales. This move indicates a market readiness for the product, despite the complexities of regulatory approval and consumer demand.
Marketing Med第一季度:两张 Lands on the制作 line. While focusing on a niche market, the drug has been notable for its marketing reach. In 2023, the ‘Viagra for women’ name became a household name, with viewership spikes across TV and digital platforms. This influx has tempted couples and菲亚米消费者 to explore the effects of this groundbreaking innovation, further 加(empowering diverse audiences.
The Modern Semis: Misinterpretation and UFO. Despite its immense success in clinical trials and TV manifestation, the name “Viagra for women” has caused a stir among both users and the public. Alternative medicine Monthly has discussed its possible uses as part of a broader pseudonym debate, suggesting potential for the drug to influence public opinion. This has led to debates about prioritizing scientific evidence rather than traditional marketing tactics, highlighting the need for a more responsible approach to medical innovation.
The World Beyond Sexual基本面: Beyond the Page. Beyond just sexual function, Myregyna has the potential to shift societal conversations in various ways. Some users have suggested that this product, while controversial, offers a glimpse into a future where individuals can experience full emotional freedom or/.
Conclusion: The Start of a New Era. For now, Myregyna stands as a testament to advancements in understanding and addressing male and female sexual health. While the name “Viagra for women” carries challenges, the product’s success and potential for broader societal impact make it a subject of both celebration and warrants improvement. This journey toả::_ Avoiding the pitfalls of the drug, it remains a fascinating exploration into the future of health and sexuality.